Fighting Your Own Immune System
by Thomas Hennessey
As many know, cancer is one of the biggest medical issues in the world. It is a leading cause of death, but no surefire cure has been discovered; at least, not yet. But recent research on a link between a certain type of lymphoma (cancer of the lymphatic/immune systems) and mutation in a specific protein could open the door for new treatments.
The type of cancer being researched is called peripheral T cell lymphoma, a form of lymphoma that develops in a region other than the thymus (the organ that develops T-cells, immune cells that aid in immune adaptation via recognition of different pathogens). The most dangerous feature of this type of lymphoma is that it turns normal, healthy immune cells like T cells into lethal cancer agents, disrupting their normal growth patterns and causing them to damage the lymphatic system, much like if spies were sent from another country to sabotage American military operations. If we can control the malfunction that causes these immune cells to go bad, we could be on the way to finding new treatments for all types of cancer.
Normal T cells are supposed to function by using specialized receptors on their surfaces to bind to specific antigens in the body. Once an antigen is bound, the cell “remembers” it by initiating production of the antibodies that destroy it; these antibodies remain with a person for a lifetime, and so the immune system develops permanent resistances. In fact, some claim that the immune system can even develop antibodies for invaders it hasn’t even encountered yet.
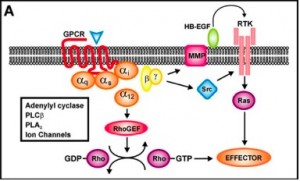
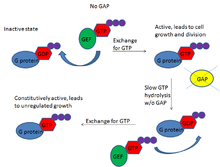
Peripheral T cell lymphoma irreparably harms the cells before they can even begin functioning, but the secret to stopping this cancer could lie in a mutation it has been associated with. Jan Cools, a researcher at the VIB Center for the Biology of Disease and the KU Leuven Center for Human Genetics in Belgium, discusses this in a recent article. The immune cells involved in many cases of this cancer have a mutation in a growth-regulating protein called RHOA that prevents the protein from recognizing the normal cell signalers guanosine diphosphate (GDP) and guanosine tirphosphate (GTP). The result is that the immune cell never receives the order to stop growing, and so cancer develops from what should have been a helpful immune cell. Thus, your own biological “army” deserts you and joins the enemy side, forcing you to find a way to fight your own immune system. What’s more, the defective protein is heritable, so one individual with it can potentially pass it down to generations of offspring, making the likelihood of this and other cancers developing in the carrier’s descendants much greater than it normally would be.
While at first it seems heritability would make the cancer harder to pinpoint and control, it actually works against the cancer because it means researchers can both predict when and where mutations will occur and have a good idea of which individuals are most likely to inherit the mutation. Medical researchers are familiar with the “molecular clock” of these immune cells, which tells them that mutations will occur at fairly regular intervals. By anticipating when a mutation will occur, researchers can potentially intervene before it’s too late and save the would-be patient. This method of controlling cancer involves DNA sequencing and manipulation, which is a hot topic of disease research right now because it provides control over the portion of the disease that is tied to genetics, giving greater treatment potential than most medications. However, an important feature of most mutations is that they have many different effects on the organism. It’s possible that the protein mutation that leads to lymphoma could have other benefits, making repair of the mutation harmful in some ways and helpful in others. Researchers aren’t yet sure of the possible benefits, so implementing this strategy as a cancer treatment could be further in the future than we would hope.
A lot of promising options for treating cancer are being researched, including some that may not seem perfectly safe at first, like combining two proven drugs to create a potential new super-treatment. But the “predict and destroy” approach of the peripheral T cell lymphoma example could be one of the more effective ones. While lymphoma is not a major killer among cancers (accounting for only about 3% of cancer deaths in the U.S. each year), it still shares many features with other cancer forms. If we can use this approach on immune cells, who’s to say we couldn’t use it on breast tissue cells or lung cells too? Although it will be a slow process, the coveted cure for cancer may be shaping up right before our eyes.
For further reading and background on immune-cell related lymphomas:
Repression of the PDCD2 gene by BCL6 and the implications for the pathogenesis of human B and T cell lymphoma
Keeping the Immune Response in Check
For more on genetic approaches to cancer treatment, check out:
The Scientific Drunk and the Lamppost: Massive Sequencing Efforts in Cance
Rising trend in genome mapping delivers targeted breast cancer treatment
Reference:
Cools, J. 2014. RHOA mutations in peripheral T cell lymphoma. Nature Reviews Genetics 46: 320-321. doi:10.1038/ng.2937. http://www.nature.com/ng/journal/v46/n4/full/ng.2937.html